

Some radionuclides have half-lives of mere seconds, but others have half-lives of hundreds or millions of years." Radioactive half-life is the time required for half of the radioactive atoms present to decay. Some decay products are a different chemical element.Įvery radionuclide has a specific decay rate, which is measured in terms of " half-life half-lifeThe time required for half of the radioactive atoms present to decay or transform. Only the final, stable atom in the chain is not radioactive. The decay products within the chain are always radioactive.
#Radioactive elements series
Also called the "decay series.".Įach series has its own unique decay chain. For example, the decay chain that begins with Uranium-238 culminates in Lead-206, after forming intermediates such as Uranium-234, Thorium-230, Radium-226, and Radon-222. The series of decay products created to reach this balance is called the decay chain decay chainThe series of decays or transformations that radionuclides go through before reaching a stable form. Those that decay in more than one step are called series radionuclides. The majority of radionuclides only decay once before becoming stable. The atoms keep transforming to new decay products until they reach a stable state and are no longer radioactive. When it decays, a radionuclide transforms into a different atom - a decay product. Elements that emit ionizing radiation are called radionuclides. Radioactive material is a class of chemicals where the nucleus of the atom is unstable. There are some elements with no stable form that are always radioactive, such as uranium. The four common radioactive elements are Uranium, Radium, Polonium, and Thorium. Unstable forms emit ionizing radiation and are radioactive. However, all elements have an unstable form. Typically, the most stable form of an element is the most common in nature. Some of these forms are stable other forms are unstable. Radioactive decay occurs in unbalanced atoms called radionuclides.Įlements in the periodic table can take on several forms. Gamma rays can pass completely through the human body as they pass through, they can cause damage to tissue and DNA. and/or gamma rays gamma raysA form of ionizing radiation that is made up of weightless packets of energy called photons. Beta-emitters are most hazardous when they are inhaled or swallowed.
#Radioactive elements skin
Some beta particles are capable of penetrating the skin and causing damage such as skin burns. Alpha particles pose no direct or external radiation threat however, they can pose a serious health threat if ingested or inhaled., beta particles beta particlesA form of particulate ionizing radiation made up of small, fast-moving particles. The ionizing radiation that is emitted can include alpha particles alpha particlesA form of particulate ionizing radiation made up of two neutrons and two protons.
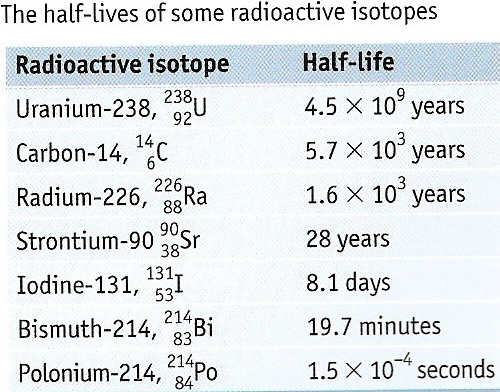
Ionizing radiation can affect the atoms in living things, so it poses a health risk by damaging tissue and DNA in genes. Radioactive decay is the emission of energy in the form of ionizing radiation ionizing radiationRadiation with so much energy it can knock electrons out of atoms.


 0 kommentar(er)
0 kommentar(er)
